Chemical Reaction and Equation
9.What are reactants and products of a chemical reaction? Explain with an example.
Answer:
The substance or the substances that take part in a chemical reaction are called reactants. The substance or the substances formed during a chemical reaction are called products.
For example, lead nitrate reacts with potassium iodide forming lead iodide and potassium nitrate. In this reaction, the starting materials namely lead nitrate and potassium iodide are the reactants. The substances that are formed during the reaction namely lead iodide and potassium nitrate are the products.
reactants and products
10.What is a chemical equation?
Answer:
The representation of a chemical reaction in the form of symbols and formulae of the substances involved in it, wherein the reactants are written on the left-hand side and the products on the right-hand side is called a chemical equation.
Check here-SSLC-Karnataka State Science Important Questions with Answer (part 2)
chemical equation
11.What is the simplest way of representing a chemical reaction?
Answer:
A chemical reaction is represented in the simplest manner using a word equation.
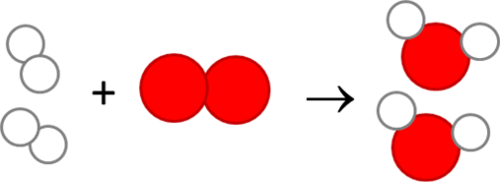
simplest way of representing a chemical reaction
12.What is a word equation in Chemistry? Explain with an example.
Answer:
A chemical reaction that is represented by writing the complete names of the reactants and products rather than their symbols or formulae is known as a word equation. It is simply a chemical equation written in words.
In a word equation, the names of the reactants are written on the left hand side and the names of the products are written on the right hand side. The reactants and products are separated by an arrow directed to the right (towards the products).
In case the reaction has more than one reactant, + sign is put between the reactants. This sign on the reactants’ side indicates ‘reacts with’ or ‘combines with’. Similarly, + sign is put between the products when there is more than one product. Here the + sign indicates ‘and’. The arrow sign indicates ‘produces’ or ‘yields’.
Consider the reaction between zinc and dilute hydrochloric acid. During this reaction, zinc chloride and hydrogen gas are formed. This can be represented by a word equation as follows

word equation in Chemistry
Check here-Karnataka State SSLC Board Science Important Questions and Answers- SSLC Tips
13.What does the arrow in a chemical equation indicate? What does a chemical equation represent?
Answer:
The arrow in a chemical reaction indicates the direction of the reaction. A chemical equation represents a chemical reaction.

arrow in a chemical equation
14.Distinguish between a skeletal equation and a balanced equation.
Answer:
1. Skeletal equation:
A chemical equation in which the number of atoms of different elements is not equal on the two sides of the equation is called an unbalanced chemical equation. It is also called skeletal equation.
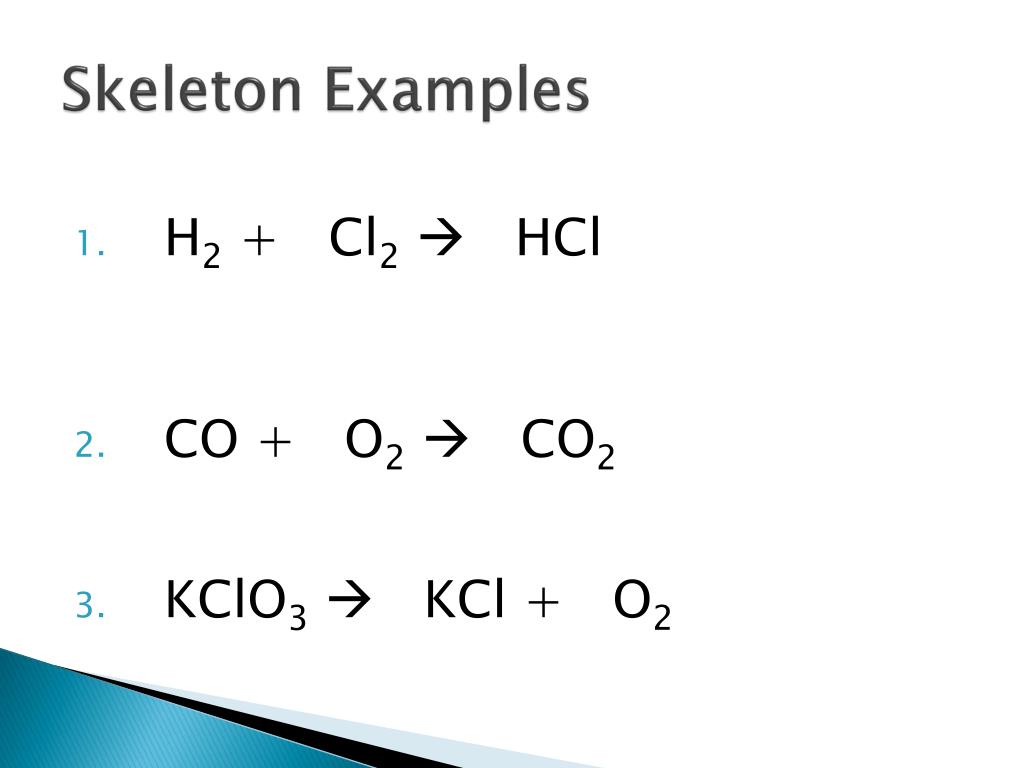
Skeletal equation
2. Balanced equation:
A chemical equation in which the number of atoms of different elements is equal on the two sides of the equation is called a balanced chemical equation.
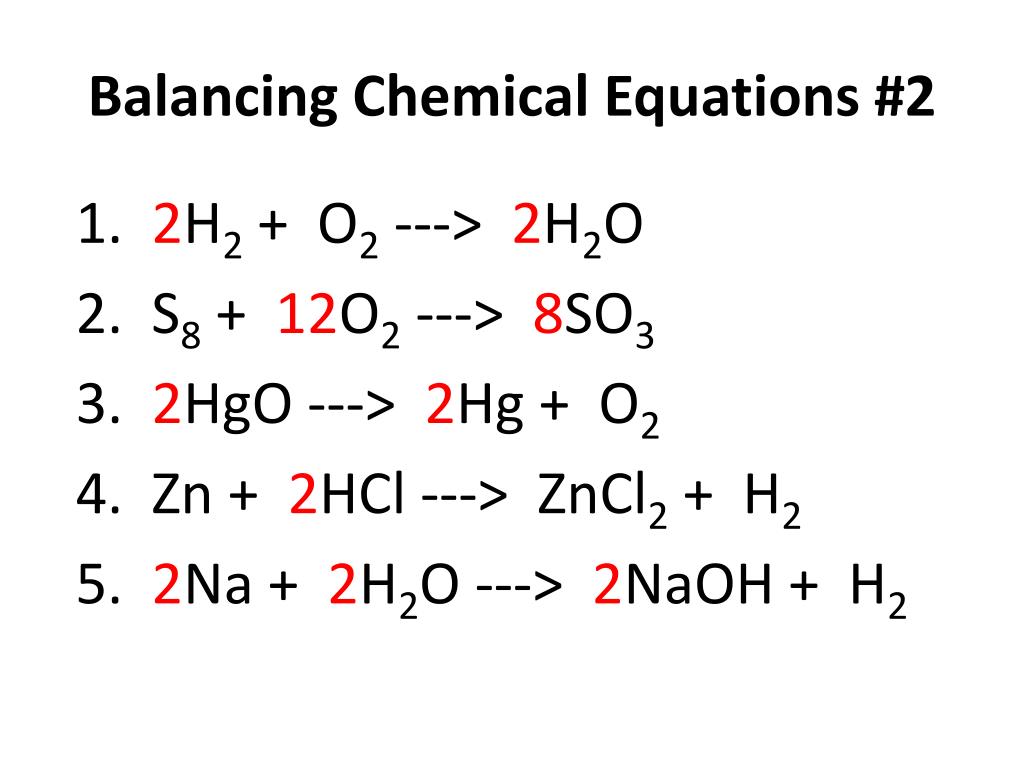
Balanced equation
Check here-SSLC Karnataka State Board Science Important Question and Answer Chemical Reaction and Equation

 Support Us
Support Us 

