Acid Basic and Salt
Q1.What happens in each of the following situations
1.Milk is left at room temperature during summers.
Ans- When milk is left at room temperature during summer season, the milk undergoes chemical change. The sugar present in milk is converted into an acid. This chemical change spoils the milk.
2.An iron tawa/pan/nail is left exposed to humid atmosphere.
Ans– When an iron material such as a nail is left exposed to humid atmosphere, the iron undergoes oxidation (a chemical change) and begins to rust. A new substance called iron oxide is formed.
3.Grapes get fermented.
Ans– When grapes get fermented, the sugar present in grapes undergoes chemical change and a new substance such as alcohol is formed.
4.Food is cooked.
Ans-When food is cooked, the components present in food undergo chemical change resulting in the formation of new substances.
5.Food gets digested in our body.
Ans-When food gets digested in our body, the various constituents of food will undergo chemical change and are broken down into simpler substances. For example, carbohydrates present in the food we consume changes into glucose during the process of digestion.
6.We respire?
Ans– When we respire, the food present in the body cells will undergo chemical change. This process produces energy and carbon dioxide.
All the reactions above involve chemical changes and in each of the changes, one or more new substances are formed.
Check here-Karnataka SSLC Mathematics Syllabus 2025-26 , Check Topic-Wise Syllabus
Q2. How do you show the formation of magnesium oxide from magnesium?
Ans-Clean a magnesium ribbon about 2 cm long by rubbing it with sandpaper. Hold it with a pair of tongs. Heat it using a spirit lamp. Magnesium ribbon burns with a dazzling white flame and changes into a white powder.
Collect this powder in a watch-glass. The ash-like powder so formed is magnesium oxide. Burning of magnesium is a chemical reaction. During this process, magnesium combined with oxygen to form magnesium oxide.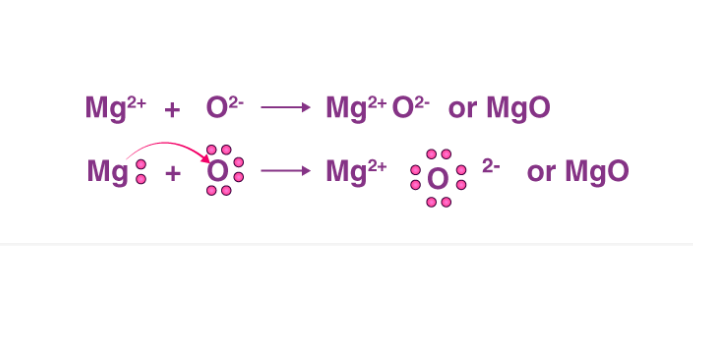
Check here-SSLC -Karnataka SSLC Science Syllabus 2025-26 With PDF, Check for Official Syllabus
Q3. Why should a magnesium ribbon be cleaned before burning in air while preparing magnesium oxide?
Ans-When magnesium ribbon is exposed to moist air, a layer of magnesium oxide is formed on its surface. This hinders the process of burning. This is why magnesium ribbon must be cleaned with sand paper before heating it while preparing magnesium oxide.


 Support Us
Support Us 

